Changing the paradigm
What if feathers originated in the Early Triassic? The evidence from feathers in ornithischian dinosaurs and in pterosaurs, while controversial, points to an origin of feathers in the ancestor of dinosaurs and pterosaurs, the so-called basal avemetatarsalian, dating minimally to the Early Triassic, 250 million years ago (Benton et al. 2019). This contrasts with the current view that feathers arose in the Middle Jurassic in the Coelurosauria, the clade that includes a bunch of small, advanced theropod dinosaurs and birds.
This isn’t just a matter of shifting the point of origin back in time, but also of rethinking the role of feathers, and the shape of one of the most famous adaptive radiations of all time, the origin and diversification of birds. The new model would emphasize the special time of the Early Triassic, when life was recovering from the most devastating mass extinction of all time, and modern ecosystems were emerging. Among vertebrates, this was a time of arms races, a quickening pace of life, and the origin of warm-bloodedness in the ancestors of birds and mammals. Let’s step through the implications.
Deep origin of feathers
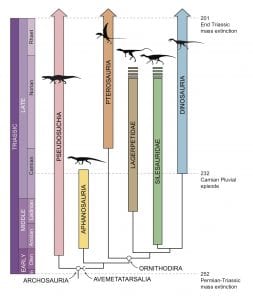 If feathers occur across dinosaurs and pterosaurs, then their origin has to be sought minimally in the Early Triassic, some 250 Myr ago. Recent evidence for the origins of pterosaurs and dinosaurs show that close outgroups of Dinosauria were already established at the end of the Early Triassic (Benton et al. 2014; Nesbitt et al. 2017), even though convincing fossils of dinosaurs and pterosaurs are not known before the Late Triassic, about 230 Myr ago.
If feathers occur across dinosaurs and pterosaurs, then their origin has to be sought minimally in the Early Triassic, some 250 Myr ago. Recent evidence for the origins of pterosaurs and dinosaurs show that close outgroups of Dinosauria were already established at the end of the Early Triassic (Benton et al. 2014; Nesbitt et al. 2017), even though convincing fossils of dinosaurs and pterosaurs are not known before the Late Triassic, about 230 Myr ago.
[Right] Evolution of archosaurs in the Triassic, showing rapid diversification of all groups in the Early Triassic, following the massive disruption of the Permian-Triassic mass extinction, the time when pterosaurs and dinosaurs diverged from their (feathered) common ancestor. [Redrafted from the work of Sterling Nesbitt.]
This then shifts the origin of dinosaurs and pterosaurs back into a time of considerable disruption in Earth and life, the 8 Myr span of recovery from the Permian-Triassic mass extinction (Chen & Benton 2012; Lau et al. 2016). Massive volcanism across the Permian-Triassic boundary, 252 Myr ago, led to a series of environmental catastrophes, including sharp global warming, acid rain, mass wasting, ocean stagnation and acidification, that drove more than 90% of species on land and in the sea to extinction.
These sharp environmental perturbations were repeated several times through the Early and early Middle Triassic, from 252–244 Myr ago (Payne et al. 2004), and the recovery of life was initiated and quashed several times. The effect seems to have been that modern-style ecosystems emerged at the end of the maelstrom, comprising ancestors of many modern groups (e.g. lissamphibians, turtles, lizards, crocodilians, dinosaurs, and mammals).
Speeding up in the Triassic
Independently, several studies have shown how medium-sized vertebrates on land had enhanced their physiological and ecological pace with higher metabolic rates and greater ability to acquire food. For example, abundant fossil trackways from around the world show a sharp shift from sprawling to erect postures across the Permian-Triassic boundary (Kubo & Benton 2009; Sullivan 2015).
Further, bone microstructure shows that Triassic archosaurs and synapsids had small cortical canals and cell lacunae, indicating they had small red blood cells and high aerobic capacity (Huttenlocker & Farmer 2017). Bone microstructure also indicates fast growth rates in Early and Middle Triassic archosaurs, just as in dinosaurs and pterosaurs, and more like those of birds than crocodilians (Ricqlès et al. 2008; Cubo et al. 2012; Legendre et al. 2016; Klein et al. 2017).
Oxygen isotopic measurements from fossil bones suggest that endothermy (warm-bloodedness) evolved in synapsids, possibly multiple times, during the Middle or Late Permian (Rey et al. 2017). Finally, dinosaurs, pterosaurs, and their ancestors all show postcranial skeletal pneumaticity, evidence for supplementary air sacs and unidirectional air flow, as in birds (Butler et al. 2012), suggesting they all had increased activity levels and endurance. These fundamental postural and physiological changes in the two great terrestrial tetrapod lineages (archosaurs, synapsids) during the Early Triassic confirm that ecosystems operated at a different pace, and high activity levels and speed were essential in the predator-prey arms races.
It is no surprise then that synapsids from the Late Permian onwards (Lovegrove 2017; Rey et al. 2017), and archosaurs from the Early Triassic onwards bore insulating pelage, whether hair or feathers. The endothermy of pterosaurs and dinosaurs, and indeed their avemetatarsalian ancestors (Butler et al. 2012), would indicate the likelihood of an insulating epidermal covering of some kind, especially in the smaller (< 1 m body length) species which could not rely on mass homeothermy.
Feather evolution
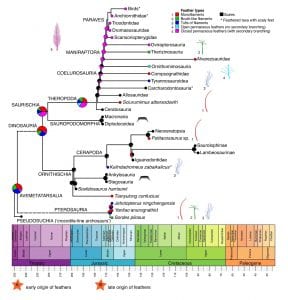
The diversification of different feather types is only understood incompletely. At present, we can suggest that pterosaurs and ornithischian dinosaurs had a variety of simple feather types, none of them with a contour or aerodynamic function, but simple monofilaments, bristles, quills, and tufted and bunched filaments, all presumably for insulation. In the ceratopsian Psittacosaurus, the quills were feather-like, but perhaps distinct from regular bird feathers.
[Right] Macroevolution of feathers. The phylogenetic tree shows the major groups of dinosaurs and pterosaurs , scaled against geological time. The tree represents a best estimation of ancestral states (shown as probabilities of different states in the pie charts) of scales and feathers throughout the phylogenetic tree, from a computational analysis (Yang et al. 2019). Each of five feather morphologies, numbered 1–5, and scales, are shown based on fossil evidence for each major group. The ancestral state reconstruction shows a combination of monofilaments, tuft-like filaments and brushtype filaments as the ancestral state for Avemetatarsalia and Dinosauria. Two hypotheses for the timing of avian feather origins are indicated: early origin, at the base of Avemetatarsalia in the Early Triassic (A) or late origin, at the base of Maniraptora in the Early–Middle Jurassic (B).
So far, feathers have not been identified in the armoured ankylosaurs or stegosaurs among ornithischians, nor in the sauropodomorphs. It will be interesting to find whether early non-armoured thyreophorans and smaller sauropodomorphs might have had feathers before they were either crowded out by the bony armour plates or the giant size of these two dinosaurian groups.
The absence of feathers in certain dinosaurs has been used as evidence that the ancestor of dinosaurs, or of dinosaurs and pterosaurs, likely did not have feathers either. The idea is that feathers arose at least three times, in theropods, ornithischians and pterosaurs, and so the absence of feathers in armoured stegosaurs and ankylosaurs and in the giant sauropods is because their ancestors never had feathers. However, the alternative view (Godefroit et al. 2014; Xu et al. 2014; Yang et al. 2019) is that the ancestor had feathers, and feathers were lost or suppressed in certain groups.
Theropods show a great diversity of feather types, and the clade Coelurosauria, originating in the Late Triassic, shows the same simple feather types as ornithischian dinosaurs and pterosaurs, but members of the clade Maniraptora (Ornithomimidae, Therizinosauridae, Oviraptoridae, Dromaeosauridae, Trodontidae, Avialae; see diagram above) show pennaceous feathers, as seen in birds.
There are steps in feather evolution then, with a variety of monofilaments and bunched filaments arising in the Early Triassic and prevailing in diverse pterosaurs and dinosaurs through to birds, and then pennaceous feathers arising in the Middle Jurassic in Maniraptora, the clade with reduced body size, elongate arms, and varied flying ability.
Literature cited
- Benton , M.J., Dhouailly, D., Jiang, B.Y., and McNamara, M. 2019. The early origin of feathers. Trends in Ecology & Evolution online before print (doi: 10.1016/j.tree.2019.04.018) ). pdf.
- Benton, M.J., Forth, J., and Langer, M.C. 2014. Models for the rise of the dinosaurs. Current Biology 24, R87–R95.
- Butler, R.J., Barrett, P.M. and Gower, D.J. 2012. Reassessment of the evidence for postcranial skeletal pneumaticity in Triassic archosaurs, and the early evolution of the avian respiratory system. PLoS One 7, e34094.
- Chen, Z.Q. and Benton, M.J. 2012. The timing and pattern of biotic recovery following the end-Permian mass extinction. Nature Geoscience 5, 375–383.
- Cubo, J., Le Roy, N., Martinez-Maza, C. and Montes, L. 2012. Paleohistological estimation of bone growth rate in extinct archosaurs. Paleobiology 38, 335–349.
- Godefroit, P., Sinitsa, S.M., Dhouailly, D., Bolotsky, Y.L., Sizov, A.V., McNamara, M.E., Benton, M.J., and Spagna, P. 2014. A Jurassic ornithischian dinosaur from Siberia with both feathers and scales. Science 345, 451-455. pdf. Supplementary file.
- Huttenlocker, A.K. and Farmer, C.G. 2017. Bone microvasculature tracks red blood cell size diminution in Triassic mammal and dinosaur forerunners. Current Biology 27, 48–54.
- Kubo, T. and Benton, M.J. 2007. Evolution of hindlimb posture in archosaurs: limb stresses in extinct vertebrates. Palaeontology 50, 1519–1529. pdf.
- Lau, K.V. et al. 2016. Marine anoxia and delayed Earth system recovery after the end-Permian extinction. Proceedings of the National Academy of Sciences, U.S.A. 113, 2360–2365.
- Legendre, L.J., 2016. Palaeohistological evidence for ancestral high metabolic rate in archosaurs. Systematic Biology 65, 989–996.
- Lovegrove, B.G. 2017. A phenology of the evolution of endothermy in birds and mammals. Biological Reviews 92, 1213–1240.
- Nesbitt, S.J. et al. 2017. The earliest bird-line archosaurs and the assembly of the dinosaur body plan. Nature 544, 484–487.
- Payne, J.L., Lehrmann, D.J., Wei, J.Y., Orchard, M.J., Schrag, D.P. and Knoll, A.H. 2004. Large perturbations of the carbon cycle during recovery from the end-Permian extinction. Science 305, 506–509.
- Rey, K. et al. 2017. Oxygen isotopes suggest elevated thermometabolism within multiple Permo-Triassic therapsid clades. eLife 6, e28589.
- Ricqlès, A. de, Padian, K., Knoll, F. and Horner, J.R. 2008. On the origin of high growth rates in archosaurs and their ancient relatives: complementary histological studies on Triassic archosauriforms and the problem of a “phylogenetic signal” in bone histology. Annales de Paléontologie 94, 57–76.
- Sullivan, C. 2015. Evolution of hind limb posture in Triassic archosauriforms. In: Great Transformations in Vertebrate Evolution (Dial, K. et al., eds.), pp. 107–124. University of Chicago Press.
- Xu, X., Zhou, Z.H., Dudley, R., Mackem, S., Chuong, C.M., Erickson, G.M. and Varricchio, D.J. 2014. An integrative approach to understanding bird origins. Science 346, 1253293.
- Yang, Z.X., Jiang, B.Y., McNamara, M.E., Kearns, S.L., Pittman, M., Kaye, T.G., Orr, P.J., Xu, X., and Benton, M.J. 2019. Pterosaur integumentary structure with complex feather-like branching. Nature Ecology & Evolution 3, 24–30. pdf. Supplementary file.

