One objection to the identification of melanosomes in fossil feathers of birds and dinosaurs has been that these organelles are unlikely to survive the fossilization process. However, as demonstrated by Jakob Vinther et al. (2008, 2010), while he was still a PhD student at Yale University, there is extensive evidence for the preservation of melanosomes in fossils. This is because the melanin molecule is resistant to degradation (Glass et al. 2012; Lindgren et al. 2012). McNamara et al. (2013) showed that melanosomes are resistant to degradation during diagenesis. These discoveries show why melanosomes are so commonly preserved and have higher resistance to decay than the keratin substrate of feathers and hairs in a variety of physical environments.
The toughness of melanin and melanosomes
 Melanin provides not only colour, but also structural strength. It strengthens structures by cross-linking of proteins (Riley 1997). Melanins supply mechanical strength to seed pods in plants and insect cuticles. Further, black hairs and are generally thicker and tougher than white or grey hair and feathers (Liu et al. 2003a, b). Extensive studies of human hair show that bleaching by sunlight or chemical means, with oxidation of melanins, substantially weakens the hair, even though melanins make up only 2% of the hair (Hoting & Zimmermann 1997). Similar phenomena are seen also in birds. For example, in birds that are primarily white, such as geese, gulls, pelicans, and cranes, the wing tips often carry a flash of black colour, a terminal set of feathers containing intense packing of eumelanosomes, in order to provide strength and protection (McGraw 2006). This idea that melanin produced abrasion resistance in feathers was proposed first by Burtt (1986), and has since been tested experimentally (McGraw 2006).
Melanin provides not only colour, but also structural strength. It strengthens structures by cross-linking of proteins (Riley 1997). Melanins supply mechanical strength to seed pods in plants and insect cuticles. Further, black hairs and are generally thicker and tougher than white or grey hair and feathers (Liu et al. 2003a, b). Extensive studies of human hair show that bleaching by sunlight or chemical means, with oxidation of melanins, substantially weakens the hair, even though melanins make up only 2% of the hair (Hoting & Zimmermann 1997). Similar phenomena are seen also in birds. For example, in birds that are primarily white, such as geese, gulls, pelicans, and cranes, the wing tips often carry a flash of black colour, a terminal set of feathers containing intense packing of eumelanosomes, in order to provide strength and protection (McGraw 2006). This idea that melanin produced abrasion resistance in feathers was proposed first by Burtt (1986), and has since been tested experimentally (McGraw 2006).
[Above] The white ibis, showing black wing tips. [Image from All About Birds.]
Surprisingly, the chemical structure of melanin is not fully resolved, in part because of its resistance to hydrolysis. However we do know that the molecule itself is highly cross-linked (and that it doesn’t just cross-link other molecules).
It is well documented (Goldstein et al. 2004; Gunderson et al. 2008) that melanized feathers are more resistant to bacterial degradation than non-melanized feathers. It has also been shown (Bonser 1995) that melanized feathers are more abrasion resistant. Liu and Simon (2003a, b) demonstrated that eumelanin is resistant to chemical degradation.
These observations are supported by extensive practical work in pigment materials research. For example, both eumelanosomes and phaeomelanosomes are routinely extracted from inside keratinous substrates such as hair using various methods of acid/ base extraction, indicating their strong resistance to chemical degradation; alternative methods using enzymatic extraction retain not only the physical structure, but alter the chemistry less (Liu et al. 2003b, and references therein).
Recent studies on the taphonomy of hair samples in archaeological contexts (Wilson et al. 2007) also show that melanin and melanosomes are resistant to decay, even more so than keratin: “Indeed, in the most severe cases of microbial destruction to the hair shaft, the complete loss of keratinaceous material was associated with concomitant survival of melanin pigment granules.”
Melanosomes, not bacteria
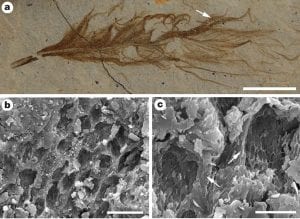
1. Melanosomes embedded in keratin structure. First, the bodies occur embedded inside the feathers, and in those feather parts that exhibit melanosomes in modern birds (Durrer 1986; McGraw 2006). In extant birds, melanosomes in the feather barbules are arranged in complex arrays (Durrer 1986). The typical configuration is one or more layers of regularly oriented melanosomes suspended in a β-keratin matrix below a superficial layer of β-keratin; melanosomes can also occur, usually arranged less regularly, medial to such layers (Durrer 1986). Preservation (presumably as primarily an organic remain) of the degraded keratinous matrix occurs locally in some of the Jehol feathers, most obviously where the fossil bodies are exposed as moulds (Benton et al. 2008); the fossil bodies are, like melanosomes, clearly embedded within this matrix, and are not a superficial coating. The integumentary filaments also exhibit this feature. These phenomena occur in the feathers of birds and dinosaurs from the Jehol Group and other localities around the world. There is likely no geological age limit, since examples from 125 and 170 million years ago can be preserved as well-defined, fully turgid, structures.
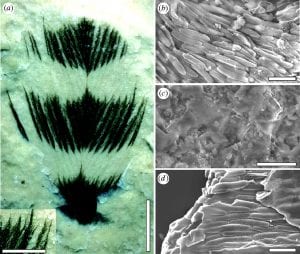 (Left) Cretaceous feather ultrastructure compared with that in a living bird. (a) Feather from the Crato Formation, Early Cretaceous, Brazil (Leicester University, UK, Geology Department, LEIUG 115562) showing colour bands; margins of colour bands are similar to those found in living birds and barbules are clearly preserved. (b) Dark bands, composed of aligned eumelanosomes, contrast with (c) light areas that reveal only the rock matrix. (d) A broken barbule from a modern Red-winged Blackbird (Agelaius phoeniceus, Aves: Icteridae, Yale Peabody Museum 1047) reveals eumelanosomes aligned along the barbule enclosed in a keratin matrix. Scale bars, (a) 3 mm, insert 1 mm; (b) 1 μm; (c) 10 μm; (d) 1 μm. [Image from Vinther et al. 2008]
(Left) Cretaceous feather ultrastructure compared with that in a living bird. (a) Feather from the Crato Formation, Early Cretaceous, Brazil (Leicester University, UK, Geology Department, LEIUG 115562) showing colour bands; margins of colour bands are similar to those found in living birds and barbules are clearly preserved. (b) Dark bands, composed of aligned eumelanosomes, contrast with (c) light areas that reveal only the rock matrix. (d) A broken barbule from a modern Red-winged Blackbird (Agelaius phoeniceus, Aves: Icteridae, Yale Peabody Museum 1047) reveals eumelanosomes aligned along the barbule enclosed in a keratin matrix. Scale bars, (a) 3 mm, insert 1 mm; (b) 1 μm; (c) 10 μm; (d) 1 μm. [Image from Vinther et al. 2008]
2. Melanosomes occur only in dark bands. Second, it has been shown (Vinther et al. 2008) that eumelanosomes occur only in the dark bands of banded feathers, and not in the light bands (see Figure left): a fossilized biofilm of keratinophilic bacteria would be likely to occur throughout a uniformly preserved structure, and not stop suddenly along an apparent feather stripe. Notably, those parts of a feather that lack melanosomes, the calamus and proximal part of the rachis, are repeatedly absent in Jehol materials unless preserved in calcium phosphate (see first Figure). There is no reason to suppose that a film of keratinophilic bacteria would have developed elsewhere over the surface of the feather, but not on these parts, nor could their absence imply that these portions were buried in the skin and so escaped bacterial replacement because most of the rachis would have been exposed. There is evidence that melanosomes can leak out of tissue structures (Glass et al. 2012).
3. Unusual packing ultrastructures. The third line of evidence for fossil melanosomes comes from Vinther et al. (2008), who showed packing and layering of melanosomes in fossil feathers, identical to ultrastructures seen in modern feathers and in the Jehol feathers, but incompatible with a bacterial origin. Further, size-specific layering of melanosomes has also been noted in internal soft tissues of fossil frogs (McNamara et al. 2018).
4. Concentration of melanin corresponds to intensity of colour. In birds and non-avian dinosaurs, their feathers may show patterns of spots and stripes, or gradually changing colour. In a study of a 55 Myr old fossil contour feather from the Fur Formation of Denmark, Field et al. (2013) noted it had a dark distal tip that graded to a lighter base. Close study by scanning electron microscopy and synchrotron-based trace metal mapping confirmed that this gradient was caused by differential concentration of melanin. In a survey of 321 modern feathers from 18 orders of birds, the investigators found the same phenomenon: graded patterns of colour corresponded to less or more intense concentrations of melanin. Such a close correlation of melanosomes in the fossils with expressed colours further confirms that the structures seen in the fossil feathers are melanosomes and not bacteria.
Experimental decay of melanin
 What if the melanin identified by palaeontologists and organic geochemists is not melanin at all; after all, it has a somewhat different structure from modern melanins. In a series of experiments, Caitlin Colleary, then a MSc student in Bristol under Jakob Vinther’s supervision, subjected samples of modern melanin to high temperatures and pressures to try to replicate the processes of fossilization. In laboratory experiments over spans of weeks, Caitlin replicated millions of years of pressure and heating in the bowels of the Earth.
What if the melanin identified by palaeontologists and organic geochemists is not melanin at all; after all, it has a somewhat different structure from modern melanins. In a series of experiments, Caitlin Colleary, then a MSc student in Bristol under Jakob Vinther’s supervision, subjected samples of modern melanin to high temperatures and pressures to try to replicate the processes of fossilization. In laboratory experiments over spans of weeks, Caitlin replicated millions of years of pressure and heating in the bowels of the Earth.
She found (Colleary et al. 2015) that melanin degrades under high temperature , but retains a recognizable chemical signature when analysed with time-of-fight mass spectrometry (Figure). In fact, the experimentally modified melanins migrated across the analytical space (see Figure left) as the temperature rose from room temperature to 200 degrees and 250 degrees. A little longer in the experimental set-up and she might well have entirely replicated the fossil melanins.
There seems little doubt that melanin is a tough molecule and it can survive all kinds of harsh treatment during fossilization (Vinther 2015. 2016) – even Cambrian chordates and vertebrates from the Burgess Shale and Chengjiang show black eye spots, evidence of melanin in the retina.
Literature cited
- Benton, M.J., Zhou, Z.-H., Orr, P.J., Zhang, F.-C. & Kearns, S.L. 2008. The remarkable fossils from the Early Cretaceous Jehol Biota of China and how they have changed our knowledge of Mesozoic life. Proceedings of the Geologists’ Association 119, 209-228. Download the paper here
- Bonser, R.H.C. 1995. Melanin and the abrasion resistance of feathers. Condor 97, 590-591.
- Burtt, E.H., Jr. 1986. An analysis of physical, physiological and optical aspects of avian coloration with emphasis on Wood Warblers. Ornithological Monographs 38: 1-126.
- Colleary, C., Dolocan, A., Gardner, J., Singh, S., Wuttke, M., Rabenstein, R., Habersetzer, J., Schaal, S., Feseha, M., Clemens, M., Jacobs, B.F., Currano, E.D., Jacobs, L.L., Sylvestersen, R. L., Gabbott, S.E. & Vinther, J. 2015. Chemical, experimental, and morphological evidence for diagenetically altered melanin in exceptionally preserved fossils. Proceedings of the National Academy of Sciences of the USA, 112, 12592–12597.
- Durrer, H. 1986. The skin of birds: Colouration. In Biology of the Integument 2, Vertebrates (eds Bereiter-Hahn, J., Matolsky, A.G. & Richards, K.S.), pp. 239-247. Springer.
- Field, D.J., D’Alba, L., Vinther, J., Webb, S.M., Gearty, W. & Shawkey, M.D. 2013. Melanin concentration gradients in modern and fossil feathers. PloS One 8, e59451.
- Glass, K., Ito, S., Wilby, P.R., Sota, T. et al. 2012. Direct chemical evidence for undegraded eumelanin pigment from the Jurassic Period. Proceedings of the National Academy of Sciences, U.S.A. 109, 10218–10223.
- Goldstein, G., Flory, K. R., Browne, B. A., Majid, S., Ichida, J.M. & Burtt, E. H. 2004. Bacterial degradation of black and white feathers. Auk 121, 656-659.
- Gunderson, A.R., Frame, A. M., Swaddle, J. P. & and Forsyth, M. H. 2008. Resistance of melanized feathers to bacterial degradation: is it really so black and white? Journal of Avian Biology 39, 539-545.
- Hoting, E. & Zimmermann, M. 1997. Sunlight-induced modifications in bleached, permed, or dyed human hair. Journal of the Society of Cosmetic Chemists 48, 79-91.
- Lindgren, J., Uvdal, P., Sjövall, P., Nilsson, D.E. et al. 2012. Molecular preservation of the pigment melanin in fossil melanosomes. Nature Communications 3, art. 824.
- Liu, Y. & Simon, J. D. 2003a. Isolation and biophysical studies of natural eumelanins: applications of imaging technologies and ultrafast spectroscopy. Pigment Cell Research 16, 606-618.
- Liu, Y., Kempf, V. R., Nofsinger, J. B., Weinert, E. E., Rudnick, M., Wakamatsu, K., Ito, S., & Simons, J. D. 2003b. Comparison of the structural and physical properties of human hair eumelanin following enzymatic or acid/base extraction. Pigment Cell Research 16, 355-365.
- McGraw, K.J. 2006. The mechanics of melanin coloration. In Bird Coloration. 1. Mechanisms and Measurements (eds Hill, G.E. & McGraw, K.J.), pp. 243-294 (Harvard University Press, Cambridge).
- McNamara, M.E., Briggs, D.E.G., Orr, P.J., Field, D.J. et al. 2013. Experimental maturation of feathers: implications for reconstructions of fossil feather colour. Biology Letters 9, 1–6.
- Riley, P.A. 1997. Melanin. The International Journal of Biochemistry & Cell Biology 29, 1235-1239.
- Vinther, J. 2015. A guide to the field of palaeo colour. Bioessays 37, 643–656.
- Vinther, J. 2016. Fossil melanosomes or bacteria? A wealth of findings favours melanosomes. BioEssays 38, 220-225.
- Vinther, J., Briggs, D.E.G., Prum, R.O. & Saranathan, V. 2008. The colour of fossil feathers. Biology Letters 4, 522-525.
- Vinther, J., Briggs, D. E. G., Clarke, J., Mayr, G. & Prum, R. O. 2010. Structural coloration in a fossil feather. Biology Letters 6, 128-131.
- Wilson, A.S., Dodson, H.I., Janaway, R.C., Pollard, A.M., & Tobin, D.J. 2007. Selective biodegradation in hair shafts derived from archaeological, forensic and experimental contexts. British Journal of Dermatology 157, 450-457.
- Wuttke, M. 1983. “Weichteil-Erhaltung” durch lithifizierte Mikroorganismen bei mittel-eozänen Vetebraten aus den Ölschiefern der “Grube Messel” bei Darmstadt. Senckenbergiana Lethaea 64, 509-527.
- Zhang, F., Kearns, S.L, Orr, P.J., Benton, M.J., Zhou, Z., Johnson, D., Xu, X., and Wang, X. 2010. Fossilized melanosomes and the colour of Cretaceous dinosaurs and birds. Nature 463, 1075-1078. Download the paper here and the Supplementary data.